All Articles

Probationary employees across the FDA received notices Saturday evening that their jobs were being eliminated.

Reps. Shontel Brown, Ayanna Pressley and Nydia Velazquez want to know why the FDA delayed its date to propose a…

The Food and Drug Administration said Thursday, Jan. 26, 2023, there are too many unknowns about CBD products to regulate…

Scientists in America are on a mission to ease the nation's transplant shortage by bioengineering replacement organs.

The Food and Drug Administration assertion comes over two years after they declared Makena ineffective, which Covis Pharma challenges.

The Food and Drug Administration is warning TikTok users not to participate in the 'Sleepy Chicken' craze, which can be…

Abbott Nutrition will pause production at a Michigan baby formula factory that had just restarted after being closed for several…

The hope is that an extra shot will shore up protection for kids ages 5 to 11 as COVID-19 infections…

It took trips over state lines, navigating roads and laws, for a South Dakota mother of two to get abortion…

The U.S. government will pay Pfizer $5.29 billion for 10 million treatment courses of its potential COVID-19 treatment if regulators…

Kid-size doses of Pfizer’s COVID-19 vaccine may be getting closer as government advisers on Tuesday began deliberating.

Kid-size doses of Pfizer’s COVID-19 vaccine appear safe and nearly 91% effective at preventing symptomatic infections in 5- to 11-year-olds.
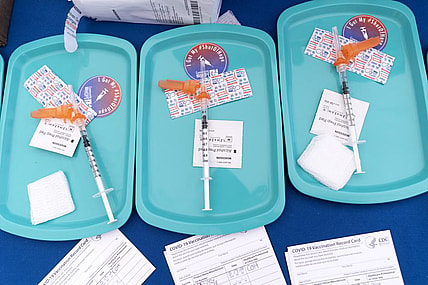
Children ages 5 to 11 will soon be able to get a COVID-19 shot at their pediatrician’s office, local pharmacy…

Johnson & Johnson asked the Food and Drug Administration on Tuesday to allow extra shots of its COVID-19 vaccine.

The FDA authorized booster doses for Americans 65 and older, younger people with underlying health conditions and those in high-risk…

The Biden administration’s embattled plan to dispense COVID-19 booster shots to most Americans faced its first major hurdle Friday.

Pfizer says it plans to meet with U.S. health officials to discuss federal authorization of a third dose of its…

Moderna said its COVID-19 vaccine strongly protects kids as young as 12, a step that puts the shot on track…

It's a step preliminary to the Food and Drug Administration authorizing the vaccine for emergency use. That could happen as…

